Manitoba limits use of AstraZeneca to reserve supply for second doses
Read this article for free:
or
Already have an account? Log in here »
To continue reading, please subscribe:
Monthly Digital Subscription
$0 for the first 4 weeks*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*No charge for 4 weeks then price increases to the regular rate of $19.00 plus GST every four weeks. Offer available to new and qualified returning subscribers only. Cancel any time.
Monthly Digital Subscription
$4.75/week*
- Enjoy unlimited reading on winnipegfreepress.com
- Read the E-Edition, our digital replica newspaper
- Access News Break, our award-winning app
- Play interactive puzzles
*Billed as $19 plus GST every four weeks. Cancel any time.
To continue reading, please subscribe:
Add Free Press access to your Brandon Sun subscription for only an additional
$1 for the first 4 weeks*
*Your next subscription payment will increase by $1.00 and you will be charged $16.99 plus GST for four weeks. After four weeks, your payment will increase to $23.99 plus GST every four weeks.
Read unlimited articles for free today:
or
Already have an account? Log in here »
Hey there, time traveller!
This article was published 12/05/2021 (1671 days ago), so information in it may no longer be current.
Manitobans who took the AstraZeneca COVID-19 vaccine are being assured they did the right thing as the province halts first doses to conserve vials for second shots amid concerns about supply.
As other Canadian provinces paused first doses of AstraZeneca/Covishield due to supply issues, and Ontario and Nova Scotia announced it will no longer offer first doses due to an increase in reports of rare blood clots, Manitoba health officials said the vaccine is safe.
“Over the last two months when we’ve been giving AstraZeneca, it has protected many tens of thousands of Manitobans who would not otherwise have had that protection,” Dr. Joss Reimer, the medical lead for the provincial vaccine task force, said Wednesday during a noon-hour news conference.
“If you got the AstraZeneca vaccine as soon as you were eligible, and I know a lot of people in the 40-and-up age category did that, you did the right thing.”
Much of the 7,530 doses of AstraZeneca/Covishield vaccine remaining in the province will be reserved for second-dose vaccination only, Reimer said. First doses of AstraZeneca can only be administered to Manitobans who have no way of getting a Pfizer or Moderna shot at supersites or pop-up clinics.
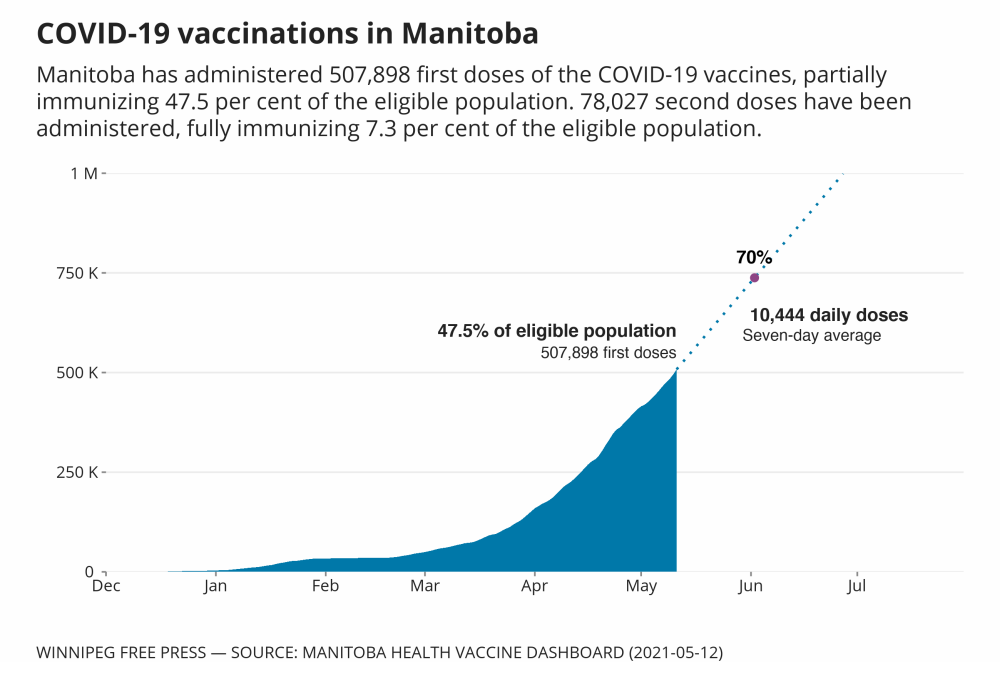
All adult Manitobans were eligible for immunization as of Wednesday.
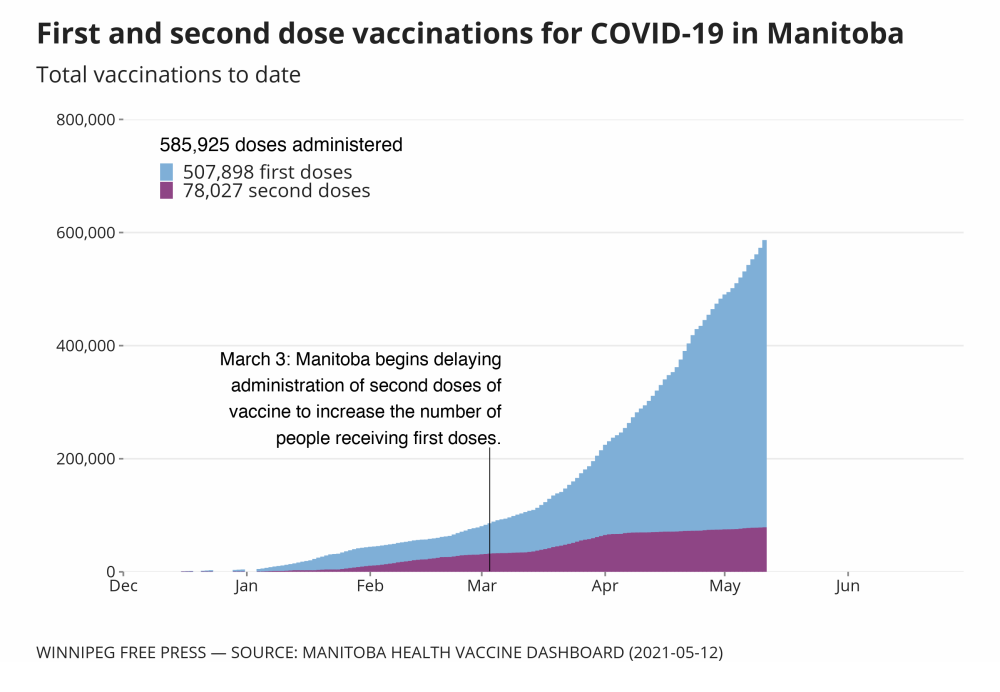
According to provincial data, about 76,700 Manitobans have received a first dose of AstraZeneca. Administration of the vaccine began March 11. A second dose of the vaccine is recommended at the 12-week mark, as it’s shown to be more effective with an extended dosing interval.
“There’s no change in our clinical guidance. We remain confident that AstraZeneca is a safe and effective vaccine,” Reimer said.
“If you got the AstraZeneca vaccine as soon as you were eligible, and I know a lot of people in the 40-and-up age category did that, you did the right thing.” — Dr. Joss Reimer, medical lead for the provincial vaccine task force
A greater supply of Pfizer and Moderna — and the uncertainties surrounding the export of AstraZeneca from India — has prompted the province to change course, she said.
The government of India has placed an export ban on AstraZeneca, which is manufactured at the Serum Institute of India.
“We want to look to the supply issues of AstraZeneca and make sure that we’re saving as much as possible, given the uncertainties we have around the export of vaccine,” she said.
Doses of the product are still expected from COVAX, a global vaccine sharing initiative that assists in procuring vaccines for lower- and middle-income countries, while discussions continue with the United States for additional shots, federal officials said last week.
Ottawa has confirmed a delivery of 23,800 doses of AstraZeneca will arrive in Manitoba the week of May 17. No deliveries beyond that date have been confirmed.
The province may end up offering a messenger RNA vaccine (Pfizer or Moderna) as a second dose to people who got AstraZeneca if supply is unavailable or if studies currently underway show a benefit to mixing vaccines, Reimer said.
“Regardless of what happens, you will be offered another dose of vaccine,” she said.
Of the AstraZeneca/Covishield doses currently in Manitoba, a portion will expire at the end of May, while the balance will expire at the end of June.
It’s unlikely any Manitobans would qualify for their second dose of AstraZeneca by May 31, based on a 12-week dosing schedule.
Over two million Canadians have received the AstraZeneca vaccine. There have been 17 confirmed cases of a rare, but severe, potential side-effect causing blood clots and low platelets called vaccine-induced thrombotic thrombocytopenia. Three women have died.
— with files from The Canadian Press
danielle.dasilva@freepress.mb.ca

Our newsroom depends on a growing audience of readers to power our journalism. If you are not a paid reader, please consider becoming a subscriber.
Our newsroom depends on its audience of readers to power our journalism. Thank you for your support.
History
Updated on Wednesday, May 12, 2021 7:37 PM CDT: Fixes image tag.